The UK launched a nationwide COVID-19 vaccination campaign starting on the 8th of this month. Except for the allergic side effects of the two vaccinated subjects, no particularly serious side effects and problems have been reported so far.
At the same time, the United States also began the most urgent mass vaccination operation since the polio vaccine in the 1950s on December 14th local time.
According to foreign media reports, the vaccination plan in the United Kingdom is going smoothly, but the situation in the United States is different. About 47% of the people say they are “unwilling to vaccinate”; moreover, the policies and conditions of each state are different, which poses a huge challenge to the vaccination process.
One week after vaccination
According to the BBC, the UK is the first country to allow mass vaccination against the novel coronavirus.
A week has passed since the vaccination began on December 8, during which no other particularly serious side effects and problems occurred except for the allergic side effects of the two vaccinated subjects. Therefore, this unprecedented vaccination work is progressing relatively smoothly.
United Kingdom: Only two people have allergic side effects
According to the British Medical Service (NHS), as of the 14th, the supply centers for COVID-19 vaccine had expanded to 100, and the vaccination targets had also expanded from the initial general medical staff to nursing home residents and people over 80 years old.
At present, 50 of these hospital-based supply centers are vaccinate in the hospital against the novel coronavirus.
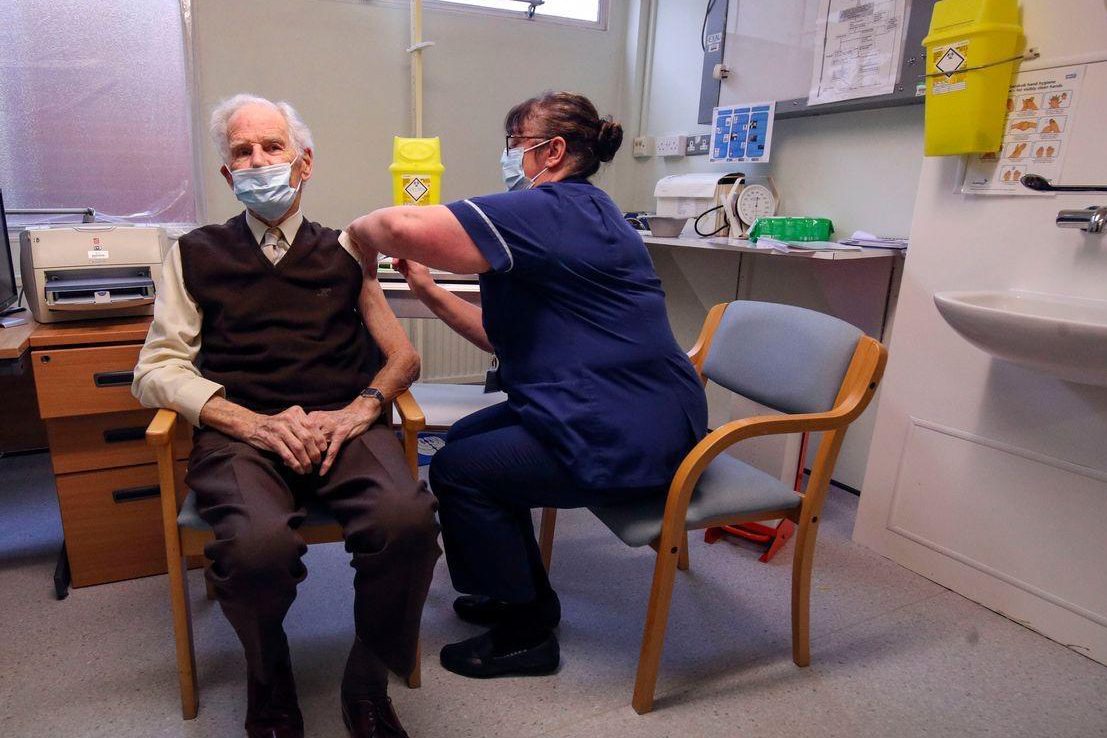
It is reported that the selection of vaccination recipients varies according to region. The England region has been vaccinated for general health care workers since the 14th, and Scotland has not decided on the specific time of vaccination for general health care workers so far. Instead, people in nursing homes are required to give priority vaccination.
It is reported that the COVID-19 vaccine vaccinated in the United Kingdom is jointly developed by Pfizer in the United States and BioNTech in Germany. The BBC reported that the fears of British citizens about getting the vaccine have almost disappeared.” Patients are looking forward to this vaccine, and very few people are worried about its side effects. A professional doctor said in an interview.
Vaccination work is rapidly carried out in the UK. According to the BBC, the COVID-19 vaccine supply center will continue to double this week from the current 100 (as of the 14th). If there is no particularly serious problem, the British government plans to expand the number of such supply centers to 1,200 within a few weeks.
At the same time, if the vaccine jointly studied by AstraZeneca and Oxford University is approved, the British government will also consider universalizing the novel coronavirus vaccine in the next stage. Now the vaccine jointly developed by Pfizer and BioNTech needs to be preserved at ultra-low temperatures of minus 70 degrees. In contrast, the vaccine jointly developed by AstraZeneca and Oxford University is more conducive to storage and circulation.
In addition, the report also mentioned that only two vaccinated subjects have had allergic side effects so far, but they have not had too much problems and are recovering. “There is no big problem with the side effects of these two vaccinated subjects, it’s just a common reaction when new vaccinations,” NHS chief Stephen Parves said in an interview. It is understood that the two vaccinated targets are both NHS staff.
The most urgent mass vaccination in 50 years
United States: There are many obstacles and the public is dissatisfied.
The Wall Street Journal reported that the United States launched the first batch of coronavirus vaccinations other than clinical trials on December 14 local time, which is the most urgent large-scale immunization operation since the polio vaccine in the 1950s. The U.S. government aims to vaccinate 100 million Americans by the end of February 2021.
But there are still many obstacles to be crossed for the U.S. government to achieve this goal. Each state’s vaccination plan and environment are different, and the transportation and custody of vaccines require huge additional budget allocations.
In addition, it is necessary to let the broad American people overcome their distrust of vaccines.
According to the Guardian, on the 13th local time, there was confusion among U.S. states about the distribution order and amount of COVID-19 vaccine. After the federal government distributes the coronavirus vaccine to the states, it is up to the states to carry out the vaccination work.
However, since specific cases have not yet been released, the vaccination programs developed by the states are different and there is a lack of a clear standard, which has led to dissatisfaction among many people.
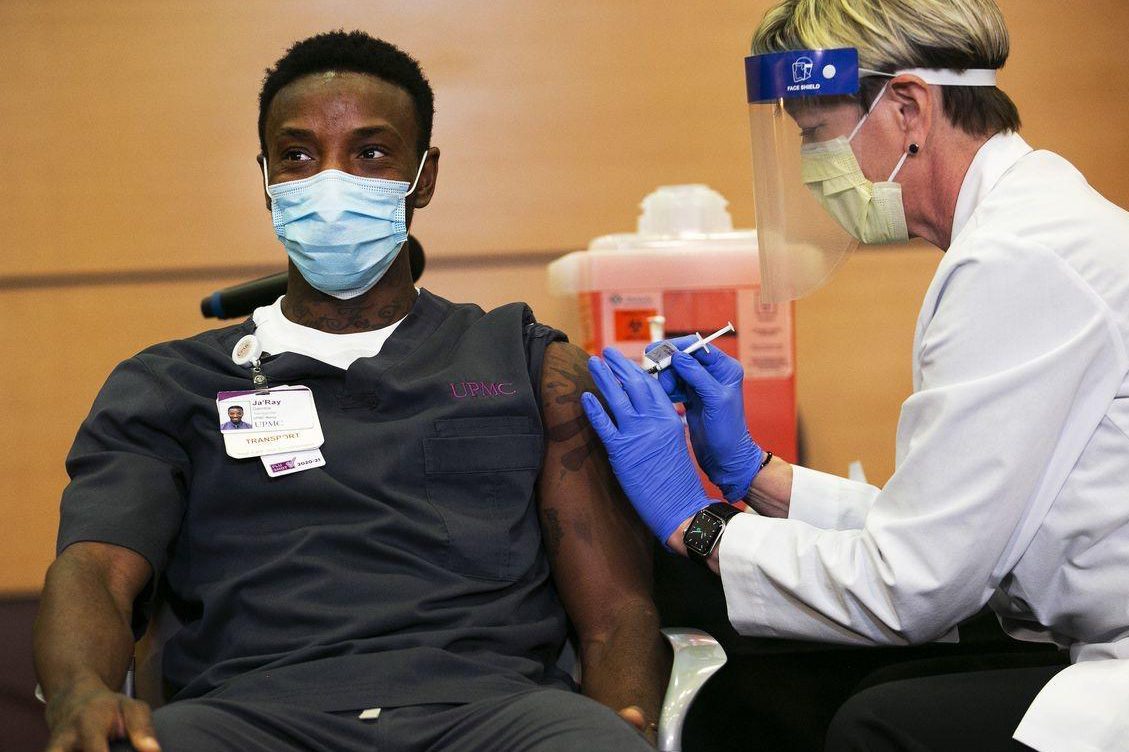
It is reported that the program of whether medical staff or nursing institutions are given priority to vaccination varies from state to state in the United States. In Kentucky, for example, the state government decided to send two-thirds of the first batch of COVID-19 vaccine to long-term care facilities, and the remaining one-third to 11 hospitals in the state for vaccination.
However, around the selection criteria of these 11 hospitals, medical stakeholders questioned it. This is mainly because many large hospitals that treat severe COVID-19 patients are not selected as the first hospitals to be vaccinated.
The opposite is true in New York State. Long-term care facilities in New York state have the most deaths from COVID-19, but even so, the New York state government has decided to give priority to vaccinations for medical staff in hospitals and other medical institutions. The decision has been heavily criticized, and “the state’s vaccination program is changing at any time, which is what caused the chaos.” The New York Times criticized.
The vaccine jointly developed by Pfizer and BioNTech was also vaccinated in the United States. Because the vaccine needs to be stored and transported at ultra-low temperatures of minus 70 degrees, this brings a new huge test to the states of the United States.
If the federal government of the United States does not support it through the budget, it is still unknown whether each state can be able to keep these vaccines.
The New York Times reported that the first batch of about 2.9 million Pfizer vaccines in the United States have been shipped one after another, and the U.S. government expects to ship 20 million doses by the end of the year.
However, the priority vaccination targets include 21 million medical staff and 3 million elderly elderly people in nursing homes. This means that the United States will no longer be able to fully vaccinate this group of people in 2020. Meanwhile, it will take months for most people in the United States to get a vaccine.
Concerned about vaccine safety
More than half of Americans are unwilling to be vaccinated.
It is worth noting that the vaccination program in the United States still faces a huge problem – how to dispel public concerns about vaccine safety?
According to a joint survey conducted by the Associated Press and NORC on December 9, only 47% of the respondents in the United States are willing to be vaccinated, but not half of them. Twenty-six percent of the respondents said they “refused to be vaccinated”, and about 70% of them refused to be vaccinated on the grounds of “concern about the side effects after vaccination”.
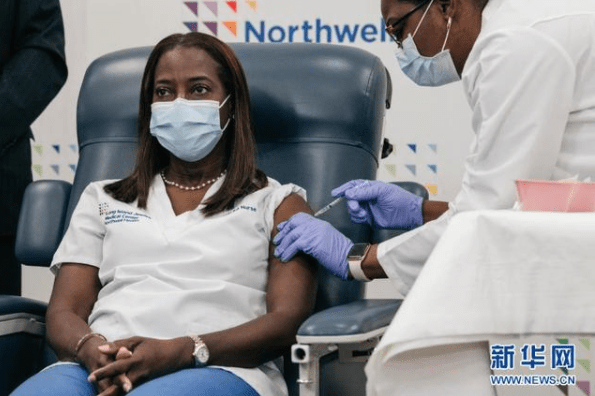
On December 14th, local time, Sandra Lindsay, a 52-year-old Jamaican intensive care unit nurse in New York, became the first coronavirus vaccinated in the United States.
The Washington Post reported that Lindsay’s identity is typical – ethnic minorities, women, and medical workers fighting on the front line of the fight against the epidemic, which made her the first vaccinated against the novel coronavirus. According to the analysis of the report, Lindsay can prove that vaccination is safe, thus dispelling doubts for more people, especially ethnic minorities.
It is reported that the death rate of COVID-19 in ethnic minorities in the United States is much higher than that of whites.
Moreover, many ethnic minorities are skeptical about vaccination and the health care system, in part because of racial discrimination and resource inequality in the American health care system.
According to the Guardian, recent data show that only 14% of black Americans said in the interview that they believed that the vaccine was safe.
It is worth noting that black Americans have nearly three times the death rate of COVID-19 as high as whites.
“To foster people’s trust in the COVID-19 vaccine, it’s not something that can be done solely by health authorities and medical experts.” Professor Li, a public health policy management at the University of New York, said that “it is necessary to transparently disclose to the general American people the whole process of the development, supply and vaccination of the novel coronavirus vaccine, and it is necessary to strengthen their knowledge and understanding of the vaccine.”