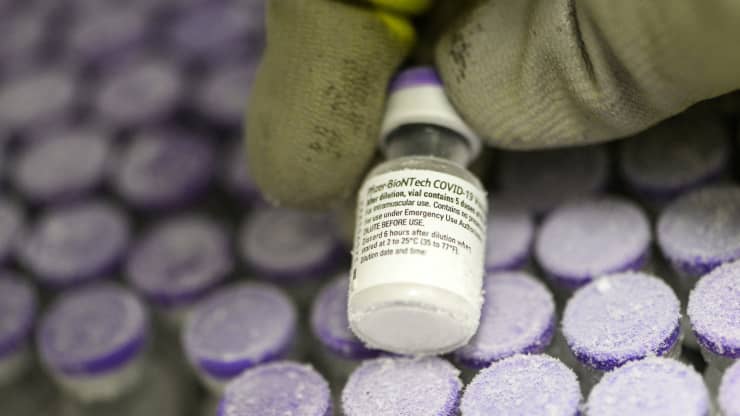
According to CNBC, on February 19 local time, Pfizer Pharmaceutical said that it was seeking the agreement of the Food and Drug Administration (FDA) to store the coronavirus vaccine it produced in the freezer of a general pharmacy for two weeks.
Pfizer and German Biotechnology (BnT) vaccines are currently extremely demanding for refrigeration and need to be stored between minus 80 degrees Celsius and minus 60 degrees Celsius.
Pfizer said it had submitted materials to the U.S. Food and Drug Administration to prove that vaccines can be stored steadily between minus 25 degrees Celsius and minus 15 degrees Celsius.
If the U.S. Food and Drug Administration approves Pfizer’s application, it will simplify the logistics of Pfizer vaccines.
“We have been conducting stability studies to support mass production of the vaccine, with the goal of making it as accessible as possible for healthcare providers and people in the U.S. and around the world,” said Pfizer CEO Albert Burla.
In a statement, he said, “If approved, this new storage option will provide more flexibility for pharmacies and vaccination centers to manage the vaccine supply.”
Pfizer’s vaccine poses logistical challenges due to the need for ultra-low temperature storage, medical experts warn.
Last December, U.S. officials said that they scrapped thousands of doses of vaccines in California and Alabama after storage temperatures were too low due to “abnormality” during transportation.
Vaccines are reported to be transported in special thermal containers, refilled with dry ice every five days for temporary storage for up to 30 days.
According to the company, vaccines can also be refrigerated for up to five days between standard refrigerator temperatures (from 2 degrees Celsius to 7 degrees Celsius) before mixing with the salt water thinner.
Whether it is Pfizer, Modena or Johnson & Johnson vaccines that have applied for emergency use rights, they all need cold chain transportation.
Pfizer said that with more stability data it expects to extend the shelf life of the vaccine and can consider using alternative short-term temperature storage.