The speed of vaccine advancement determines the future of mankind’s confrontation with the coronavirus epidemic.
The faster the vaccination speed is, the more effective the epidemic can be controlled.
The speed of virus mutation is difficult to keep up with the speed of disease prevention and control.
But in the process, the coronavirus vaccine
Factors such as protection effectiveness, production capacity, vaccination willingness, etc.
It will affect the achievement of the ultimate goal.
Who will be the first to get herd immunity when the global vaccine is launched?
The latest statistics show that an average of about 2.41 million coronavirus vaccines are injected into people’s arms every day.
According to the transmission coefficient of COVID-19, herd immunity can only be established when about 70% to 80% of the population is vaccinated, and the transmission of the virus will be blocked.
In order to achieve this goal, an unprecedented vaccination campaign is now being carried out on a global scale.
In response, Zhang Wenhong, director of the Department of Infectious Diseases of Huashan Hospital of Fudan University, pointed out that the speed of vaccine promotion determines the future of mankind’s confrontation with the COVID-19 epidemic.
The faster the vaccination speed is, the more effectively the epidemic can be controlled, and the speed of virus mutation will be difficult to keep up with the speed of disease prevention and control.
However, in this process, the protection effectiveness, production capacity, vaccination willingness and other factors of the novel coronavirus vaccine will affect the achievement of the ultimate goal.
According to Chen Xi, an associate professor at Yale University School of Public Health, China needs nearly 1 billion people to complete vaccination to meet the threshold of herd immunity.
As of January 13, 2021, China has been vaccinated with about 10 million doses of the coronavirus, but relative to the target of 1 billion people, this is only the beginning. And vaccination in Europe, the United States and other countries is not as expected at present, and new challenges have emerged.
There is no need to judge the winner or loser by the effectiveness of the vaccine.
Chinese vaccine players are beginning to enter the harvest period.
On December 31, 2020, it was officially announced that the inactivated coronavirus vaccine of the China Biological Beijing Institute of China Pharmaceutical Group had been approved by the State Food and Drug Administration on the evening of December 30th.
The results of the three-phase clinical trial showed that the vaccine’s protective effectiveness against diseases caused by COVID-19 infection was 79.34%.
The vaccine of the Chinese Medicine Institute of Medicine Beijing is the fourth in the world to release data on the effectiveness of three phases of clinical trials, followed by another inactivated coronavirus vaccine in China.
The vaccine producer Kochen carried out three clinical studies in Brazil, Indonesia, Turkey and Chile, but Brazil participated the largest number of participants in the trial, so its efficiency data will be more representative.
However, the release of Kochen’s clinical trial results in Brazil has been repeatedly delayed, the first time on December 15, 2020, and the second time on December 23, 2020.
On January 12, the Brazilian partner Butantan Institute in Kochen announced that the Kochen vaccine protection rate was 50.4%, down from 78% announced a week ago.
At the press conference, the person in charge explained that 78% of the efficiency is the result of counting mild, moderate and severe cases.
If very mild cases (reported to be symptomatic but did not go to the doctor), the figure is 50.4% – 167 infections in the placebo group and 85 in the vaccine group.
January 17th local time, Brazil’s National Health Supervision Authority authorized emergency use permits for the vaccine in Brazil.
In response to China Newsweek, Kexing said that the subjects in the Brazilian clinical trial were all medical staff, and the risk of infection was high.
The placebo group observed that the density of COVID-19 was five times higher than that of the general population in Brazil during the same period. The trial showed mild sore throat, runny nose, weakness, etc.
Symptoms are also captured, so there are many mild cases in infected people who do not need any treatment.
Jin Dongyan, a professor and virologist at the School of Biomedicine of the University of Hong Kong, told China Newsweek that medical staff are more careful. Once the infection is likely to be very careful or tested more frequently, it is possible, so that more cases can be caught in the vaccination group than in other vaccine clinical trials, and the efficiency is rare. Released.
However, “The third phase of clinical trials are supposed to be carried out in high-exposure and high-risk areas, so that it is possible to test whether the vaccine is protective and how effective it is.” A scientist involved in the development of a coronavirus vaccine pointed out.
The Kochen vaccine has initially reported different values in different clinical trials, with a protective effect of 91.25% in Turkey, 65.3% in Indonesia and 50.38% in Brazil.
Kochen explained that although the protective efficacy of each trial varies in numerical terms, the common result is that the vaccine has a clear protective effect, especially for moderate and severe symptoms.
For example, in Brazil, all seven hospitalizations and severe cases occurred in the placebo group; in Turkey, all six hospitalizations occurred in the placebo group, and the vaccine was 100% effective in the protection of hospitalization and severe cases.
Cui Xiang, who works in vaccine research and development in the United States, explained that the significance of the Kochen vaccine can be simply understood: with the vaccine, the risk of contracting the novel coronavirus has been reduced by 50%, the risk of going to the hospital due to the novel coronavirus has been reduced by 80%, and the risk of developing serious illness has been reduced by 100%.
Several experts believe that the effectiveness of the same technical route should not be very different.
It can be seen that the efficiency of the two mRNA vaccines of Pfizer and Moderna is about 95%.
As for why there are relatively big differences between the two inactivated vaccines of China’s biology and Kexing, we need to see the original data.
While the Chinese are paying close attention to the effectiveness of domestic vaccines, the data of a star vaccine abroad have also caused controversy.
January 4, Peter Dorsey, deputy editor of the BMJ’s News and Opinion team, said on the journal’s blog website that he did not think the Pfizer vaccine was effective at 95%, but only 29%.
The reason is that he believes that 3,410 suspected COVID-19 patients among the volunteers in the clinical trial may have a large number of false negative nucleic acid tests, so they should be counted as infected people, and the above results can be recalculated.
Zhu Fengcai, a clinical vaccinology expert and deputy director of the Jiangsu Provincial Center for Disease Control and Prevention, pointed out that this algorithm is unreasonable and extreme, because the symptoms of suspected patients may come from other diseases.
If there is no nucleic acid test positive, the infection with the novel coronavirus cannot be diagnosed. Among the 3,410 suspected cases, the proportion of real infection is not high. If according to Dorsey’s calculation, the protective effectiveness of all vaccines will now be less effective.
Cui Xiang told China Newsweek that the inference that questioning Pfizer’s “29%” protection effectiveness is unreasonable, but Pfizer’s clinical trial design also has something to be considered.
For example, in the double-blind design, the placebo group of the Oxford/AstraZeneca vaccine in the United Kingdom was given recombinant adenovirus, but the transmission was not the coronavirus gene, which was more rigorous.
However, the placebo group of Pfizer vaccine was given physiological saline, with fewer adverse reactions, so it was easier for researchers to distinguish which vaccines and which were No, the experiment is not the standard double-blind state.
Fengcai also mentioned that the vaccine group may have more mild symptoms than the control group, and if this group does not report, the researchers will not know that they have been infected, and the final number of infected people will be low, and the efficiency of the vaccine will be overestimated.
Although the protection data of vaccines attract the highest attention, as one pharmaceutical industry practitioner said, there is no need to regard the effectiveness as a winning or losing problem.
As long as the vaccine proves useful, it has the potential to help us control the epidemic. At present, the most urgent problem is vaccination, vaccination, vaccination.
Challenges to herd immunization in China
Recently, there have been cluster epidemics in many parts of the country.
Zhang Style, a distinguished professor of epidemiology at the School of Public Health at the University of California, Los Angeles, suggested that in view of the current difficult task of prevention and control, China could consider emergency COVID-19 vaccination against people who tested negative at the outbreak point and the surrounding population at the same time of large-scale testing to form a “security belt” to curb The virus spreads outward.
How many people need to be vaccinated for a specific population to establish herd immunity?
According to a November 2020 article published in the medical journal The Lancet by some scholars at Imperial College London, assuming that the protection of vaccines is permanent, the vaccination rate required to establish herd immunity is 60% to 72 percent at its effectiveness rate; when the vaccine is 80%, this proportion It is 75% to 90%; if the vaccine is less efficient than 80%, everyone needs to be vaccinated.

Wang Bin, inspector of the Disease Prevention and Control Bureau of the National Health Commission, said at a press conference held by the State Council on the 13th that the key groups inoculation at this stage are high-risk people aged 18-59.
With the continuous improvement of vaccine clinical research data, the need for prevention and control work and the increase of vaccine supply, China will expand the target population of vaccination to more people, including people aged 60 and above.
Huang Yanzhong, a senior researcher in global health at the U.S. Council on Foreign Relations, analyzed to China Newsweek that domestic vaccines cannot now cover people under the age of 18 and over 60, especially those at high risk, which is not conducive to the establishment of herd immunity.
According to data released by the National Bureau of Statistics, at the end of 2019, the number of elderly people aged 60 and above in China reached 254 million, accounting for 18.1% of the total population.
Another factor affecting vaccination rate is the willingness to inoculate. In October 2020, an article on the website Nature surveyed the potential acceptance of the coronavirus vaccine in 19 countries and found that China had the highest acceptance rate at 90%.
However, Tao Lina, a former immunological planning physician of the Shanghai Center for Disease Control and Prevention, said that due to the good prevention and control of the epidemic in China, many people do not think it is necessary to vacculate again.
“Now it’s not about whether to use it or not, or whether to wait for a better vaccine to accelerate vaccination.
Now it is facing the problem of supply.” Yu Li, a vaccine presiding officer who used to work for the FDA, told China Newsweek that as long as there is a vaccine, it should be vaccinated to prevent local epidemic outbreaks such as Hebei and Heilongjiang now.
By 2021, according to the production capacity plan of some domestic vaccine research and development enterprises, China’s coronavirus vaccine production capacity will exceed 2 billion doses.
Kochen has completed the construction of a second production line, and it is expected that the annual production capacity of its coronavirus vaccine will reach 1 billion doses after it is put into use in February this year.
China Bio plans to supply 1 billion doses or more of vaccines to China in 2021. According to Consino Biotechnology, it plans to build a production capacity of 100 million to 200 million doses of adenovirus vaccine this year; Fosun Pharmaceuticals and BioNTech have reached an agreement that once the mRNA vaccine developed by the latter is approved by the market in mainland China, it will supply at least 100 million doses to mainland China this year.
“There is still a certain gap, but not everyone needs to be vaccinated immediately.
Taking a step-by-step, hierarchical vaccination strategy, I think it may be solved slowly by the end of this year.” Zhang Yuntao, vice president of China Biology of China Pharmaceutical Group, said.
In the transportation and storage process, inactivated vaccines in China are more convenient than mRNA vaccines used in Europe and the United States.
Yuntao said that the storage conditions of inactivated vaccines are 2 °C to 8 °C, which is consistent with the storage and transportation conditions of all existing vaccines in China.
Cold chain transportation conditions such as Pfizer’s vaccine need to be stored at -70 °C and Moderna vaccine in the United States are -20 °C. Cold chain transportation conditions are not currently available in many cities in China, including Beijing.
“If vaccination in the United States moves as planned, herd immunity can basically be achieved around June and August, and the situation in Europe is similar.
If China does not meet this goal in time and effectively, the epidemic may last for a long time. Zhang style said.
Global vaccination speed competition
Because of various reasons such as economic strength and efficiency, the current global vaccination competition has different speeds of competition.
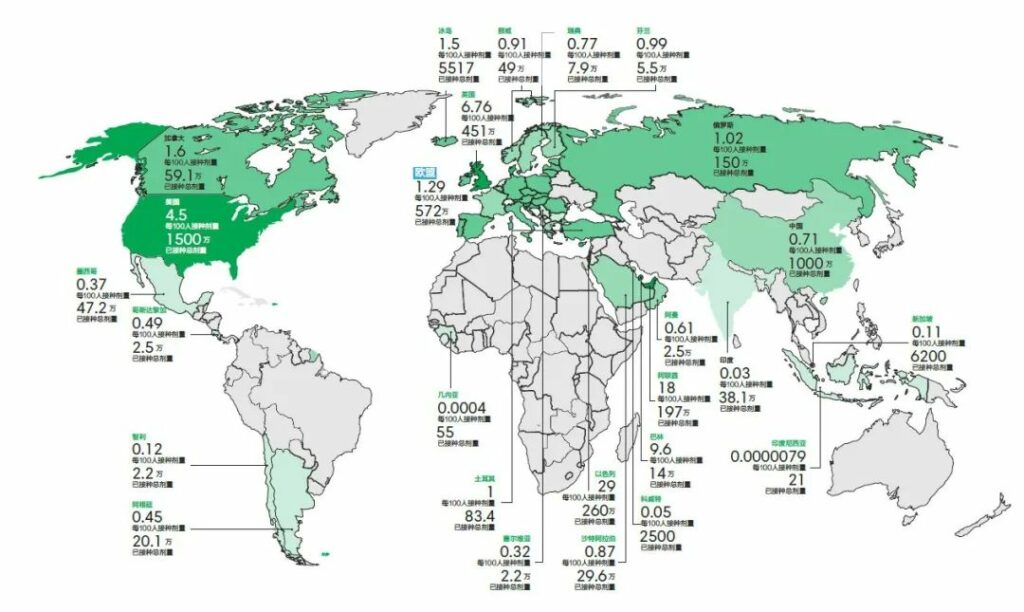
At a time when most countries in the world had not received the first injection, Israel is currently far ahead with 28.52% vaccination rates, followed by the United Arab Emirates (18.34 percent), according to the latest statistics from Bloomberg and other institutions on January 16.
The United States, China and the European Union ranked among the top three inoculation doses, but in terms of vaccination rates, the United States (4.48%), the European Union (1.29%) and China (0.71%) ranked 5, 17 and 37 in order.

Israel uses the American Pfizer vaccine and Moderna vaccine. Based on their 95% efficiency, the vaccination rate required to achieve herd immunization ranges from about 65% to 75%.
In this way, it may be the first country to achieve it. There are many reasons for Israel’s rapid progress, such as adequate supply, a country with a population of only 8.8 million, ordered 8 million doses of COVID-19 vaccine from Pfizer last November.
On the other hand, Israel has carried out large-scale public health mobilization, so the public’s willingness to vaccinate is high.
In the United States, where the epidemic is most severe, since the two vaccines were put into emergency use in mid-December last year, the most critical problem is how to spread vaccinations faster.
The United States hopes to complete 100 million vaccines in the first 100 days of 2021, that is, 1 million a day, but it has not yet been reached.
As of January 8, the agency had distributed more than 22 million doses of vaccine to states and other jurisdictions, while at this point, only 6.7 million people had completed the first dose of vaccination, according to the Centers for Disease Control and Prevention.
Originally, the United States was aiming to inoculate 20 million doses of vaccine by the end of 2020. It can be seen that the promotion efficiency is much slower than expected, including reasons such as catching up with holidays, early hesitation inoculation, and unclear states about who can be vaccinated.
Anthony Fauci, the chief infectious disease expert in the United States, believes that the United States could have done better in terms of vaccination speed. “[The goal] is clear and there is no excuse. I think we must wait until the first few weeks of January to determine what went wrong and whether we
can meet the goals set at the beginning.
To accelerate the move, on January 12, the U.S. Department of Health and Human Services has informed states to immediately expand the pool of vaccinated populations to include all elderly people aged 65 and above and vulnerable people with basic diseases, and vaccines will be distributed according to the number and the rate of vaccination of the elderly population by each state, rather than the state’s population.
At the suggestion of the federal government, some states have expanded the scope of vaccination, and thousands of people are applying for vaccination.
But these states found that they were in a new dilemma: vaccines did not arrive as scheduled, and the government actually did not have excess stocks.
Because of confidence in supply and the belief that the three-week interval between the two injections can replenish production capacity, Operation Warp Speed, which is responsible for distributing the vaccine, has stopped stocking a second dose of Pfizer/BioNTech vaccine at the end of 2020, and the last inventory of Moderna vaccines on the weekend of January 16. It is already on the transportation route.
Because of the widespread spread of mutant viruses, Chris Whitty, the chief medical officer of the United Kingdom, said that as many people at risk should be protected by vaccines as possible in the shortest possible time.
The latest data from January 16 showed that the UK ranks fourth in the world with a vaccination rate of 6.67%.
Originally, the interval between two doses of Oxford/AstraZeneca and Pfizer should be four and three weeks, but on December 30, 2020, the British government notified that the time of the second vaccine was extended to 12 weeks later.
In the current situation of vaccine shortage, in order to expand coverage, Denmark, Germany and other countries are also considering adjusting the vaccination procedures and delaying the second injection in order to expand coverage.
Lu Mengji, a German Chinese virologist and professor of the Institute of Virology of the School of Medicine of Essen, pointed out that generally speaking, after the first injection, the immune response in the body is activated, and then gradually recovers to the original state after at least three to four weeks. This is an immune cycle, and then the second injection is inoculated to strengthen long-term immunity.
Therefore, the Pfizer and Moderna vaccines have chosen a 21-day and 28-day interval, respectively.” 21 and 28 days are the lower limit, which cannot be less than this time, but it can be pushed back.
Theoretically, the more delayed the vaccination, the better the immune enhancement effect of the second injection will be. He said.
Chen Zhengming, a professor at Oxford University, said that there is also some debate in academia about whether vaccination can be delayed, but for Britain, it is really a helpless move.
The UK’s outbreak is now close to or even surpassing the first wave in early 2020 in terms of new infections, hospitalizations and deaths.
Moreover, the mutant strain spreads quickly, and the longer the epidemic lasts, the more likely more new variants are. In such an emergency situation, it is necessary to expand the coverage of vaccination as soon as possible to seize the spread of the mutant virus.
However, Sumia Swaminathan, chief scientist of the World Health Organization, recently pointed out that even if the vaccine begins to protect the most vulnerable groups, the world will not achieve any degree of herd immunity by 2021.
It may be achieved in a few regions or countries, but it is not global. She urged people to continue to practice strict social distancing this year.



