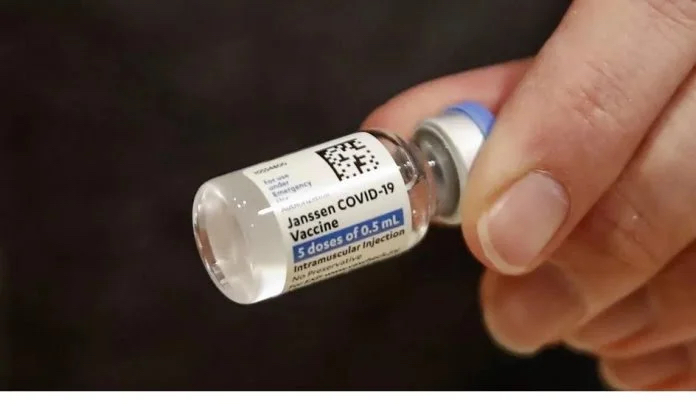
Following the AstraZeneca vaccine, the coronavirus vaccine produced by Johnson & Johnson in the United States has also been investigated by the European Drug Administration.
The European Drug Administration announced on April 9th local time that four abnormal thrombosis with thrombocytopenia have been found among the inoculated people with Johnson & Johnson vaccine, and one of them died as a result.
It is understood that one of the four thrombosis cases occurred at the clinical trial stage of the vaccine, and the other three occurred after the approval of vaccinations began in the United States.
At present, Johnson & Johnson vaccine is only inoculated in the United States. Although the European Union approved the market on March 11, 2021, the vaccine has not yet been delivered.
The European Drug Administration has made it clear that it is not clear whether thrombotic cases are associated with Johnson & Johnson vaccine.