April 7th local time, the European Drug Administration issued a statement saying that the AstraZeneca coronavirus vaccine may cause “very rare thrombosis symptoms”. But it has repeatedly said that AstraZeneca vaccine is “risk-free” and has no connection with thrombosis.
The beginning and end of the “thrombotic” disturbance of AstraZeneca vaccine
In the past month, the controversy over the AstraZeneca coronavirus vaccine has hardly stopped. A picture shows the context of the “thrombus”.
The “thrombotic” crisis continues to ferment. Many European countries “brake sharply”
In February this year, two Austrian women suffered from blood clotting disorders after being vaccinated against AstraZeneca, and one of them died. On March 7, the country announced a moratorium on vaccinations. As of March 11, the EU had received 30 reports of thrombus after vaccination. Since then, Denmark, Norway, Sweden, Italy and nearly 20 other countries have successively announced the suspension of vaccination.
AstraZeneca, supported by two major institutions, restarted after a few days of suspension of vaccination.
On March 14, AstraZeneca issued a statement saying that “there is no evidence that the risk of a vaccine caused by the company to increase symptoms such as thrombosis”. On March 18, the European Drug Administration released the results of the study, believing that the AstraZeneca vaccine was not related to the increased risk of thrombosis inoculated people, and continued to recommend vaccination. WHO also gave similar recommendations. From the 19th, France, Germany and dozens of other countries resumed vaccination.
Scientist: Linked European Drug Administration: No evidence
In late March, scientists in Germany and Norway released the results of the study to prove the link between AstraZeneca vaccine and thrombosis. On 31 March, the European Drug Administration reiterated its position, emphasizing that there are no risk factors for vaccination against AstraZeneca and that the link to the thrombus has not been “confirmed”.
Thrombotic death is still occurring, and people’s willingness to inoculate is declining.
Since April, Germany, the United Kingdom, Spain and other countries have received reports of thrombosis and death after being vaccinated against AstraZeneca. Meanwhile, polls show that the willingness of European people to get vaccinated has declined.
European Drug Administration changed its mouth. Many countries adjusted their vaccination plans.
On April 7, the European Drug Administration and the World Health Organization issued statements saying that vaccination against AstraZeneca may be “related” to thrombosis. Since then, many countries have announced the adjustment of AstraZeneca vaccination plans.
They once strongly supported AstraZeneca, but now they have changed their words one after another.
In this disturbance, it is not only the European Drug Administration that has changed its attitude towards AstraZeneca vaccine.
The European Drug Administration issued a statement on March 18 that AstraZeneca vaccine will not increase the overall thrombosis risk of inoculated patients, and there is no evidence that the emergence of thrombosis cases are related to AstraZeneca vaccine.
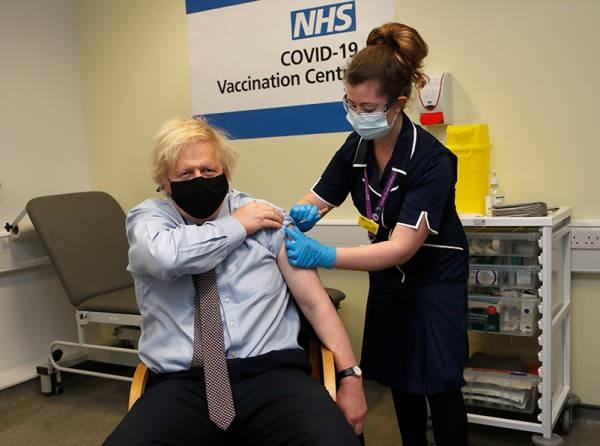
△ On March 19, British Prime Minister Boris Johnson was inoculated with the first dose of AstraZeneca coronavirus vaccine.
British Prime Minister Johnson based on the conclusions of the European Drug Administration, publicly inoculated the AstraZeneca vaccine the next day, “corrected” and called on the public to “trust the AstraZeneca vaccine, please vaccinate it as soon as possible to get protection”.
With both the European Drug Administration and the WHO insisting that getting the AstraZeneca vaccine “has more benefits than disadvantages”, many countries resumed vaccinations after a suspension of less than a week, and even quarreled over the availability of vaccines for a time.
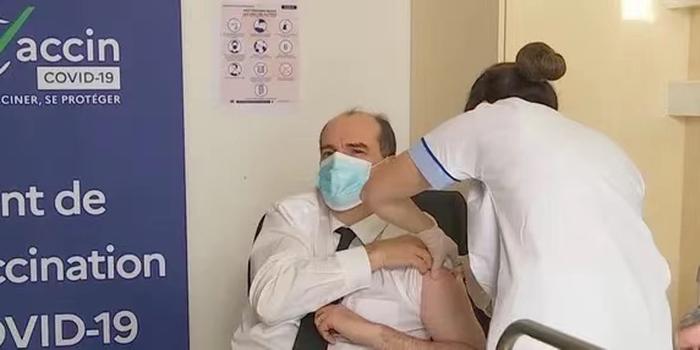
△ French Prime Minister Jean Castel was vaccinated against AstraZeneca on March 19.
French Prime Minister Castel not only immediately announced the resumption of vaccination, but also took the initiative to “show that we are all fully confident in the vaccine”. However, the country’s regulator announced on March 26 that AstraZeneca vaccine has a risk of causing atypical thrombosis, and there have been two deaths.
Because of “thrombotic risk is higher than expected”, Merkel, who said she wanted to “queue up” for AstraZeneca vaccine, had to announce a moratorium on the use of the vaccine for people under the age of 60; while the French health department said that it would allow people to switch to other brands of vaccine when taking the second dose; Canada, which once strongly supported AstraZeneca’s Canada, the Netherlands and others The country has also announced an age limit for vaccination.
The suspicion of “thrombus” is unknown. People’s concerns about AstraZeneca vaccine have intensified.
Although the cause of thrombus is still unknown, after the European Drug Administration changed its mouth, many countries announced that they would adjust the vaccination plan to raise the age of AstraZeneca vaccine.
The British drug regulator proposed on the 7th to suspend the AstraZeneca vaccine for people under the age of 30. The European Drug Administration has previously said that most of the cases of thrombosis after vaccination occur in women under the age of 60. Oxford University also announced on the same day that it would suspend the AstraZeneca vaccine experiment for children and adolescents.

In response to the new regulatory guidelines, Italy, South Korea, Spain, Belgium and others have announced the adjustment of the age setting of people vaccinated against AstraZeneca. Meanwhile, people’s doubts about the AstraZeneca vaccine are deepening.
Nearly 60 percent of French people say they don’t trust the AstraZeneca vaccine, according to the latest poll by the Elabe Institute. According to a survey by TV2 in Denmark, one-third of people in Denmark do not want to be vaccinated. The Associated Press reported that Europe is already the most skeptical continent in the world about AstraZeneca vaccine.
Compared with other vaccines approved by the European Union, the cheaper and easy-to-store AstraZeneca vaccine was once regarded by many European countries as the most effective means to achieve herd immunity. Now the “thrombus” disturbance continues to ferment, and the attitude of regulators has reversed. The change of the vaccination plan will undoubtedly deepen the suspicion and worry of the public. Art Kaplan, a bioethicist at New York University, said that the AstraZeneca vaccine mystery may cause more harm than side effects.


