The Turkish Department of Medicine and Medical Equipment issued an announcement on the 13th local time to approve the emergency use of the Kelifo coronavirus vaccine of Beijing Kexing Zhongwei Biotechnology Co., Ltd. in China. This is Turkey’s first coronavirus vaccine approved for emergency use.
The announcement said that in order to respond to the COVID-19 epidemic and ensure that people can be vaccinated quickly, the Turkish Ministry of Health approved the emergency use of Chinese vaccines.
The announcement said that the authorization was adopted on the basis of scientific data analysis and 14 days of analysis and monitoring after the arrival of the vaccine in Turkey.
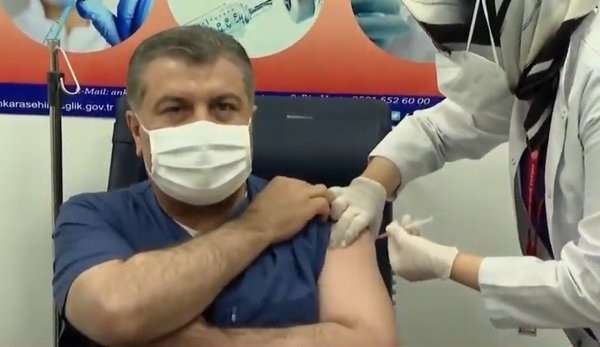
Turkish Health Minister Koja said at a press conference on the same day that medical workers across Turkey will be vaccinated against China from the 14th.
After the press conference, Koja himself and the members of the Scientific Committee specially formed to fight the epidemic were vaccinated and broadcast live to the public.
On December 24 last year, Koja held a press conference and said that the results of phase III clinical trials conducted in Turkey since September 14 showed that China’s vaccines were safe and effective.
According to the test data, the effectiveness of Chinese vaccines is 91.25%. Turkey plans to vaccinate nearly 9 million people, including medical workers, and he will also be the first batch of vaccinations.
It is reported that the first batch of 3 million doses of coronavirus vaccine ordered by Turkey from China’s Kexing Company arrived in Ankara, the Turkish capital, in the early morning of December 30, 2020.